The combination of sulfonamides and trimethoprim has a synergistic activity against numerous bacteria. Sulphonamides and trimethoprim have bacteriostatic properties, but their combined administration results in a bactericidal activity.
Veterinary products containing sulfadiazine / trimethoprim for use in drinking water:
- are rapidly absorbed
- demonstrate a high oral bioavailability (80 - 90%)
- are distributed widely throughout tissue
However, optimal clinical results can only be achieved when both active components reach the target tissue at the optimal ratio and concentration.
Poor solubility of trimethoprim
Trimethoprim is a poorly water-soluble antimicrobial with a maximum solubility of 0.4 mg/ml. The subsequent sedimentation of trimethoprim after product administration in highly concentrated stock solutions (proportioners) jeopardises the efficacy of water-soluble products containing sulfonamides and trimethoprim. If only the sulfonamide part is dissolved, animals don't benefit from the synergism, resulting in a lack of clinical efficacy. Furthermore, sedimentation in the water delivery systems creates extra work for the farmer and increases the risk of blockages in the pipelines.
HydroTrim®
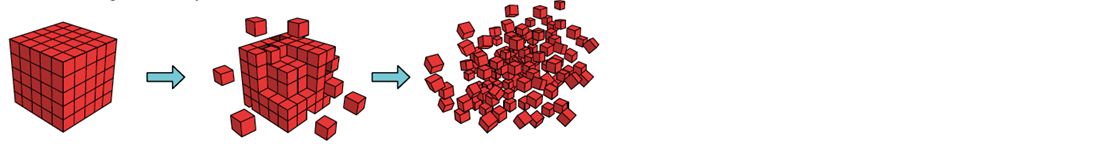
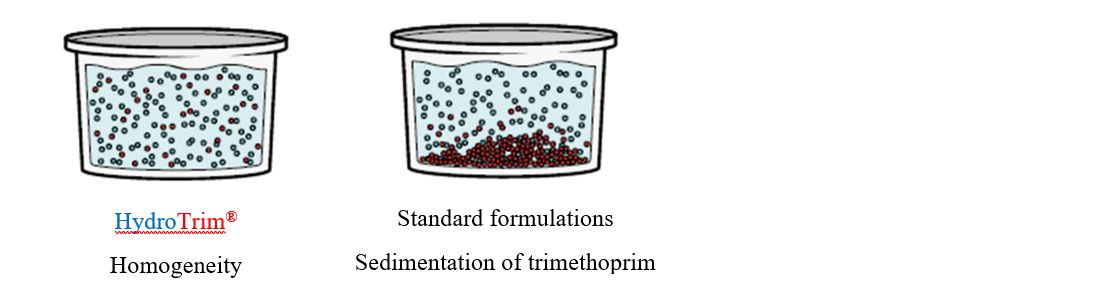
HydroTrim 500 mg sulfadiazine and 100 mg trimethoprim/g is an innovative formulation based on an advanced nanonisation technology. By using this technology, poorly soluble trimethoprim crystals are ground into many small nanoparticles (Figure 1). The subsequent increased surface area results in the fast and complete dissolution of trimethoprim, ensuring an improved efficacy based on a higher synergistic effect with sulfadiazine (Figure 2).
Solubility trial
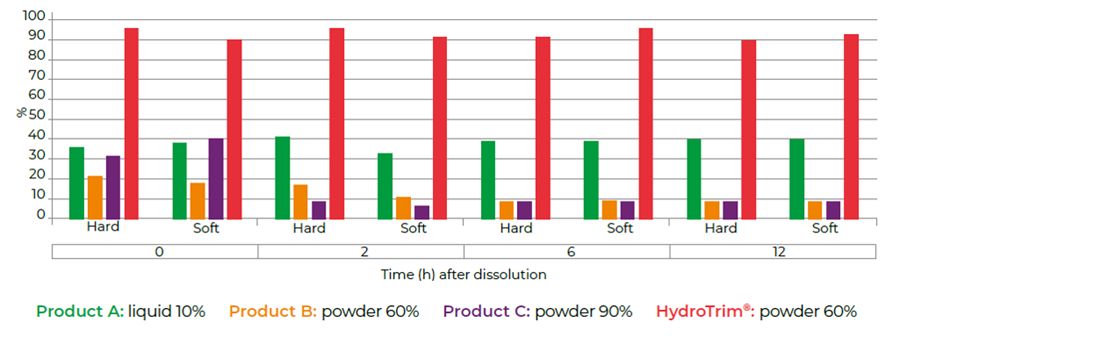
- Four water soluble commercial products containing sulfadiazine and trimethoprim at a ratio of 5:1 were evaluated.
- Samples of highly concentrated stock solutions (proportioners) were taken at different time points after product administration. The trimethoprim concentration in the drinking water of different qualities (hard or soft) was determined by high-performance liquid chromatography analysis (Figure 3).
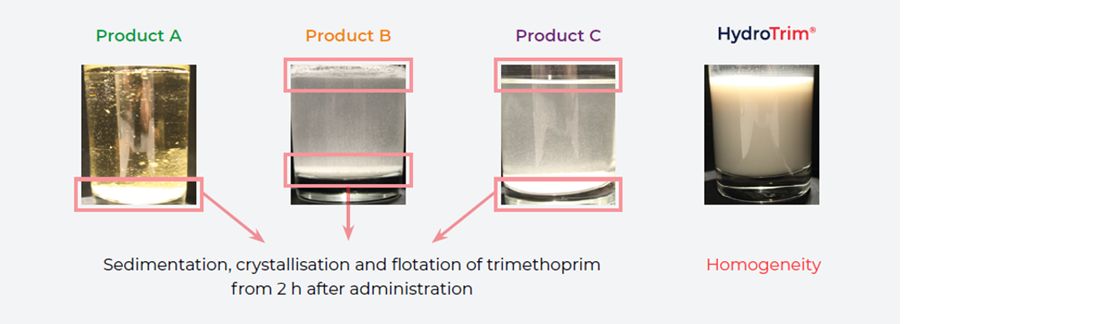
- The stock containers were also visually observed at 0, 2, 6 and 12 hours after product administration (Figure 4):
- Sedimentation, crystallisation and flotation were observed in products A, B and C from 2 hours after product administration in both water conditions.
- HydroTrim remained homogenous at all time points in both hard and soft water.
Conclusion
The nanonisation technology used in the manufacture of HydroTrim 500 mg sulfadiazine and 100 mg trimethoprim/g ensures a superior solubility of trimethoprim, even in highly concentrated stock solutions (proportioners). This results in an extended broad-spectrum activity and an improved synergistic clinical effect.

To find out more about HydroTrim, download the brochure by clicking here.


